Monday, October 30, 2006
Sunday, October 29, 2006
P&P this Thursday!!!
Friday, October 20, 2006
Thursday, October 19, 2006
Tuesday, October 17, 2006
Tuesday, October 10, 2006
Monday, October 09, 2006
Saturday, October 07, 2006
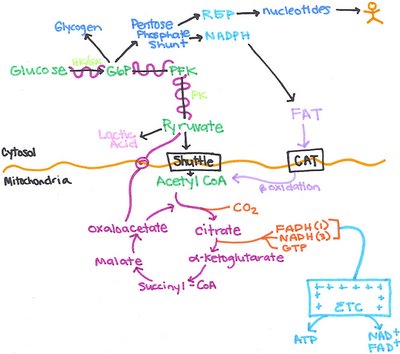
pharmaceutics review for test #1
Thursday, October 05, 2006
pharmaceutics notes
Drugs – agents to treatment, cure, diagnosis, prevention, migration (to lessen the symptoms)
Pharmacology – how these drugs work in the body
Medicinal chemistry – organic “chemical structural of the body”
Pharmaceutics - drugs are compounded and manufactured
Early Drugs Sources, types
Plants – Vinca Rosea
i. Vinrinstine
ii. Vinblastine
Animals – Premrin Esterà
Fungi/Bacteria – antibiotic
Human – Human Genome Project
Asprin (acitylcitic acid)
-Antipyretic – hypothalamus regulate body temp
-Antinflamitory – inhibit prostaglandin
-Analgesic – regulate pain
- Inhibits platel aggregation
- 50 to 75% bioavalible
USP/NF – reference book used by pharmaceuticals & pharmacists giving an indication of the quality of drugs to be used
The Federal food and drug and cosmetic act of 1938 est.
Precursor to the FDA
Formation of the FDA was caused by the sulfanilamide crisis
i. Diethylene glycol à Killed 100 people
Drug Product recall
Class 1 – very bad
Class 2 –bad
Class 3 not so bad (label, packaging error)
Drug Delivery Routes
Oral route
Transdermal – patch on skin
Parenteral – away from Gi tract (injection)
Sublingual – under the tongue (drugs that bypass the liver)
Topical – delivery of site (ointment , creams)
Ocular – treat the eye
Pulmonary – use lungs as delivery system
Other routes – rectal, vaginal, otic, nasal, urethral suppository
New Drug Development
Sources of new Drugs – pipeline
Lead compounds – protypes of new drugs
Solublity – must have aquess solubility
Absorption– movement of drug across a biological membrane
i. Drugs in salt form easily dissolved in water
ii. Amines – compounds that form when fish and animals die the smell is stopped by using lemon (it switches the amine to a salt)
Toxicity – does it have adverse reactions
Early formulation studies
Solubility
Partition coefficient –lipophilic or hydrophilic
Dissolution rated – time and how much foes into dissolution
Physical form- physical characteristics of drug
Stability – so degradation into harmful substances
Approval process
INDA – investigational new drug application
Phase 1 – main objective is to determine the safety of the drug. 20-100 patients are in this test. They are healthy volunteers that get paid. They will just be given the drug itself at a 1/10 dose. Then the drug is ramped up. And Pharmacokinetics (pharmaceutics –math, drugs, blood level of drugs)
Phase 2 – 100 to 500 patients is the drug useful “efficacious - successful in producing a desired or intended result; effective”
Phase 3 – Take the drug into large scale population 500-5000 patients
Phase 4 – clinical still occurring but it is still in use by the public
NDA – New Drug Application
Clinical studies happen over a 10 year period
Generic vs. Trade name
Patents – last 20 years (once approved about a 14 year window)
ANDA – abbreviated new drug application
Bioequivalence – do the generic provide similar blood levels
Drug Dosage and Terminology
Dosing regimen – how dose much and how long (when)
Usual adult dose – differ for adult weights
Usual pediatric dose – differ for children weights
Priming dose/loading dose –Going to pre load their blood w/drug
Maintenance dose – maintains effective blood level of drug
Prophylactic dose – tetnis shot – prevention of disease state
Therapeutic – treats disease state larger dose and longer tie frame
Drug Dosage and terminology
Therapeutic dose
i. Units of activity – biological essay use to standardize drugs
ii. MEC – minimal effective concentration is highly dependent on the drug
iii. MTC – minimal toxic concentration is highly dependent on the drug MTC you see side effects
iv. Body Weight – dosage weight based on weight of patient usually 70kgs 150lb
v. Body surface area (BSA) m2
Excipients – important ingredients
Acidifying agent used in liquid preparations to provide acidic medium for product stability “Citric acid”
i. Stabilizes the drugs
1. DuoNEB – inhaler ingredients are stable at low pH
2. Albuterol Ipotromenium – soluble @ low pH
Alkalinizing agent-used in liquid preparations to provide alkaline medium for product stability. “NaOH” “Sodium Bicarbonate”
i. Alkaline increases pH
Absorbent – an agent capable of holing other molecules onto its surface by physical or chemical means. “Activated Charcoal”
i. Holds surface exampled activated charcoal
Antifungal preservative – used in liquid and semisolid preparations to prevent the growth of fungi.
i. Examples – Butylparaben, methylparaben, proplyparaben, benzoic acid, sodium benzoate
Antimicrobial preservative – used in liquid and semisolid preparations to prevent growth of microorganisms.
i. Examples – benalkonium chloride , benzethonium chloride, benzyl alcohol , thimersol, pheylmercuric nitrate, phenol, cetypridinium chloride
ii.
Antioxidant – used to prevent deterioration of preparations by oxidation
Acorbic acid, ascorbyl palitate, sodium ascordate, sodium bisulfate, sodium metabisulfite
Buffering agent – used to resist change in pH in formulation
Potassium phosphate monobasic, sodium acetate, sodium citrate
Emulsifying agent – used to promote and maintain the dispersion of an emulsion such as a cream
Acacia, cetyl alcohol, glycery monostearate, sorbitan monooleate
Humectant – used to prevent drying of preparations, particularly ointments and cream
Glycerin, propylene glycol
Levigating agent-liquid – used as an intervening agent t reduces particle size of a powder by grinding, usually in a mortar.
Mineral oil, glycerin, propylene glycol
Suspending agent – viscosity increasing agent used to reduce the sedimentation ate of particles in a suspension
Agar, bentonite, carbomer, hydroxypropyl cellulose, hydroxypropyl methylcellulose, methlcellulose, tragacanth
Tonicity agent – used to render solutions similar in osmotic pressure to physiologic fluids, in ophthalmic, parenteral, and irrigation fluids
Sodium chloride
Preservatives – prevent the growth of bacterium or virus they do not kill they only prevent 138 -141
Sterilization and preservation Sterilization is the cleaning out of harmful substances and preservation is the stabilization of the compound to maintain current status
Static versus cidal Static prevents growth and cidal kills what is there
How to select dependent on what outcome is disered
General consideration
Modes of action
Percentages
Pediatrics - some chemicals are not safe for small children benoalcohol Not good for use in neonatal children modes of action page 140 in book
Definition of solubility –concentration of that chemical at saturation
Why should pharmacist know about solubility – drugs to be affective must be dissolved to be used by the body
How can it impact the dosage form and route of delivery –therapeutic outcomes/toxicity
Hormones are lipophilic and so you need a none water base solvent like oils and gelatin
Cases
Morphine salt is more easily dissolved than regular morphine
Creak it more potent because it is lipophilic so it is going though the body brain barrier but harder to get in a dosage form.
Solute –chemical trying to dissolve (salts have significantly higher water solubility)
Solvent – medium that you dissolve in
Factors influencing solubility smaller particle size, molecular weight, heat, adjuration, changes in pH can add and take away protons to make easier to dissolve
Chemical properties – molecule size
Pressure – high pressure means higher solubility
TERMS OF SOLUBLITY
Extremely Sol.
i. Very sol. <1>10,000
Chemical properties of the solute and solvent
Rule of likes dissolves likes
i. Inorganic are more water soluble because they many are polar
ii. Organ
1. Polar functional group –amine (NH2) Carboxyl (COOH) Methester, Hydroxyl (OH)
2. Molecular weight (higher molecular weight then higher solubility
3. Branching of chain – easier to fit objects of similar shape
Solute Crystal Structure
Unit cell shape of crystal
Polymorphism (polymorphs) – different structures the chemical could take
i. Solubility is dependent on the polymorph
Enantiotropic – many drugs can go though different polymorphs & can exist in all the different polymorphs
Monotropic – polymorphs will retrograde back into the original most stable form
Temperature
Increasing temp. Could result in water evaporation and distort the original structure of the crystal
pH
pH of GI tract
pH influence on solubility is influenced by chemical structure
Dissolution
Solubility Enhancement
Co-solvent – helps promote solubility of drug in solution
Particle size – decrease size increase rate of absorption
Pro drug –Chemically inactive compound that our body converts to active form
Surfactant (soap and dirt) – add it drug to soluablize the drug
1. (micel)
L.A.D.M.E
Liberation – liberation of drug from dosage form
Absorption – rate at which the drug goes into solution and absorbed
Distribution – once drug is in blood stream & distribution throughout the body
Metabolism – drug is chemical change in the body for excretion
Excretion – removal of chemical form body
Diffusion – of a chemical compound movement due to concentration gradient & molecular activates (random movement of molecule)
Bromian movement – particle movement/vibration
Skin – several layers of cells (topical/transdermal deliverer)
GI tract – signal layer of cells direct drug absorption
Entering a cell to induce a response
Physicochemical – molecular weight, solubility
Physiological / biological – pH gastric emptying
Dosage form / excipient – ingestion it will pass into the small intestine and will begin to be absorbed
Most drug absorption in the body occurs by passive diffusion
Flick’s law – mathematical expression that describes the diffusion process
J=dM/(Sdt)
Diffusion rate is the amount per area per time = flux
Flux is the amount of material (M) flowing through a cross-section of a barrier (S) in unit time (T).
Flux is calculated using fick’s law
J = D[(C1 – C2)/h]
D is the diffusion coeffient area per time (constant)
C1 is the concentration inside
C2 is the concentration outside
C1 –C2 is the concentration gradient
h is the thickness of the membrane
D is proptional to the chemical properties(drug + solvent), temperature and pressure
Things that will affect J
i. As C2 approachs C1, J will decrease
ii. If h increases, J will also decrease
Typical permeation Profile
Flux is the amount of drug pass per unit of time
Lag time is the time it takes to reach concentration gradient
Dissolution
Nayes Whitney equation
i. Dm/dt = DS (Cs – C)/h
ii. This means if you increase surface area then you will increase the rate of diffusion
Physicochemical Factors
Lipophilicity –
Ionization –
Solubility
Stability
Food –
Exipents - exipent affects what amount of products are going to be absorbed
MW of drug
Co administration of drug
Physiological / Biological Factors
Structure of GIT
GI pH
Gastric emptying time
Intestinal mobility – slows down with age
Blood flow – slows with age
GI mucin and bile
Disease state
Species
Physicochemical Factors Lipid Solubility
The higher the lipid solubility of a drug the easier it passes across cell membrane
Dissolution & Diffusion is give and take with lipophilic and Lipophobic
i. Lipophilic – hard to get in dissolution but easier to diffuse
ii. Lipophobic is easier in diffusion but harder to diffuse
iii. In organic molecules they are lipophilic that are hard to diffuse
iv. Dissolution is the rate limiting factor
Ionization
If the drug is ionized it is more water-soluble. Meaning it will pass though the membrane more slowly
If unionized it is less water soluble and will diffuse faster
Partition coeffient - the ratio in which phase the drug is present
To calculate the lipophilicity/hydrophilicity measure the partion coefficient
Most drugs are absorbed in the small intestine
Salt form increases stability of pharmaceutical products while increasing solubility and dissolution rates
History – world wars led to shift toward dispensing role of pharmacist
Compounding is very contriveral because the FDA and pharm. Industry and media are against it because it competes with them and their profit
Allows independents to competes with chain because it offers a niche to them that the others can not offer
1994 compound was less than 1% in 2002 it is 8%.
Why the increase? Pharmacists were getting tried of strictly dispensing and it offered patient cumstomablity of their drugs
Increased compound Pharm. Care Movement
Individual patient care could not always be met by industry
i. Like low volume product shortages and low profits items
Home health care – IV home infusion, independents could use it as a profit
Branches of Compounding
Basic
Hormone Replacement – supplement hormones for patient when their hormone levels decrease (Premrin)
i. Many new compounding triest (E1, E2, E3)
1. E1- estrone
2. E2 estradiol
3. E3- esterol
Vet – cash only with a wide variety
Respiratory Therapy – many nebulizors for asthma
Sterile Products – monitored by USP 797
i. Many legal issue with people not using sterile techniques
Legal
i. Triad relationship – with is the patient doctor and pharmacist
ii. Compounding without a prescription is considered manufacturing
iii. If you compound you must use USP ingredients
iv. Compounding – mixing, assembling, packaging, or labeling a drug or device
Look at logs
Why is pH important
Drug stability
i. Affects absorption
ii. Amoxicillin in water will undergo hydrolysis when in an alkaline solution (basic)
1. Stable at pH 5.6 to 6.4
2. Vancommycin – is most stable pH 3 to 5
pH can change solubility which can inturn cause drugs to come out of solution
Drug that precipitate out of solution it is normally adsorbed to the IV tubing
Drug pH determine drug stability, solubility, absorption, charge binding to receptor
Bronsted-Lowery = acid is a proton donor and base is a proton acceptor
Lewis – acid is an electron pair acceptor, base is an electron pair donor
Protophilic – capable of accepting protons from the solute
Progenic – proton donating solvent
Amphiprotic – acts both as proton acceptor and proton donor
Aprotic solvent – neither accepts nor donates a proton
Tuesday, October 03, 2006
helpful tips for using our google group
First thing first: our website is
Below is the page you will see when you visit the above link. (I know the picture is probably bad, but it is the best I could do.)
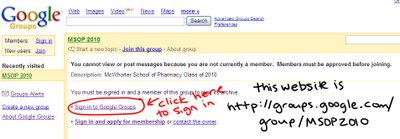
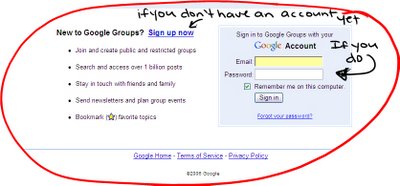
If you have not yest created your google account, you must before you can access the group's page. So click SIGN UP NOW. If you have not received an invitation to the group, or get a message saying that you must be added email me (dmchares@samford.edu)
If you do have an account already just sign in.
You will then be directed to our group's home page!
 I know this picture is bad, but hopefully you can get a general idea of the layout.
I know this picture is bad, but hopefully you can get a general idea of the layout.
Your information is featured at the top, along with a list of your groups, preferences of your account, help, and sign out shortcuts.
If you want to post you can click on Start a new topic, and you will be directed to a page that is set up for posting.
OR
If you want to post with an attachment simply send an email. It is really simple, here's how:
You can post photos or files to your group by emailing them as attachments to your group's email address. When doing so, please keep the following in mind:
- the size limit for attachments is 1MB
- you can only post attachments to a Google Group (not to a Usenet newsgroup)
- you can only post attachments via email (not through the GoogleGroups interface)
Once your message is in your group's archive, it will stay there as long as your group exists or until you remove it. Also, we don't offer a specific file storage system, so you'll need to find the message with the file in order to view it.
OUR GROUP EMAIL IS: MSOP2010@googlegroups.com
Here are some helpful help topics:
Getting started
Participating in a group
Troubleshooting
Also if you want to take a tour of google groups just click here!
I hope this helps everybody. Our group is going to be a great help to us. I will be linking a lot of helpful downloads from the group to this blog. SO please check the blog regularly. You can post comments or questions directly on this blog by clicking the comments link below. You do not have to sign in to post a comment, but if you do not sign in please put your name at the end of your comment so that we know who you are. Blog uses the same sign on name and password as the google group, so really everyone should be able to post a comment showing your name. Try it out and see how it works!
ANNOUNCEMENTS FOR WEEK OF 10-2
Two class members will be invited to attend each meeting. We will randomly draw names out of a hat and contact you by email the week prior to the meeting. You can decline the invitation if you want.
Pictures for the class directory will be this Thursday. 10/5
We will be preparing for a can food drive during Thanksgiving, so start collecting you cans and other nonperishable foods.
The white coat ceremony is this Friday at Shades Mt Baptist Church on Columbiana road. We need to be seated with our coaters by 130 that afternoon.
Keep an eye out for a card for Joe, and if you want to help him out during the weeks to come contact Emily Morris.
Things due this week:
Professionalism Paper Due today
SNPhA 10/6 see Karleta
APHa ($55) 10/6 see Emily Morris
White Coat Ceremony 10/6 seated by 130 @ Shades Mt BC
NCPA ($10) 10/3 meeting at 10am 2nd year room
name tag money due last week Emily Morris
The officer meeting went very well yesterday. Look forward to many great things to come!
If you want any announcements please contact your friendly secretaries.
Frances fvcoheno@samford.edu
Delia dmchares@samford.edu